
Batch & Release Documentation Automation
Achieve clinical manufacturing excellence & compliance
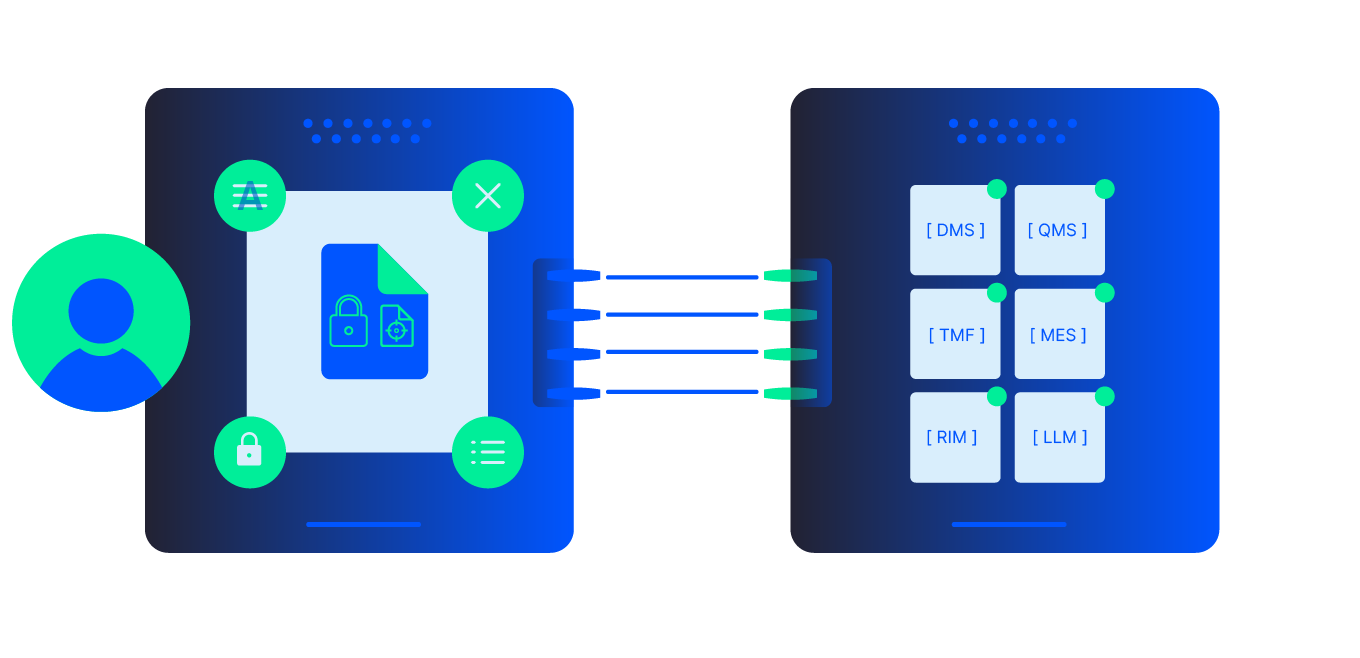
Adlib automates the transformation of batch production records, validation protocols, SOPs, and quality documentation into structured, AI-ready, audit-ready content. By normalizing formats, validating against technical criteria, and integrating with QMS and MES, Adlib reduces manual QC, lowers recall and deviation risk, and accelerates batch release across highly regulated manufacturing environments.









.jpg)
