
Clinical Trial Management Automation
Automate clinical trial document processes
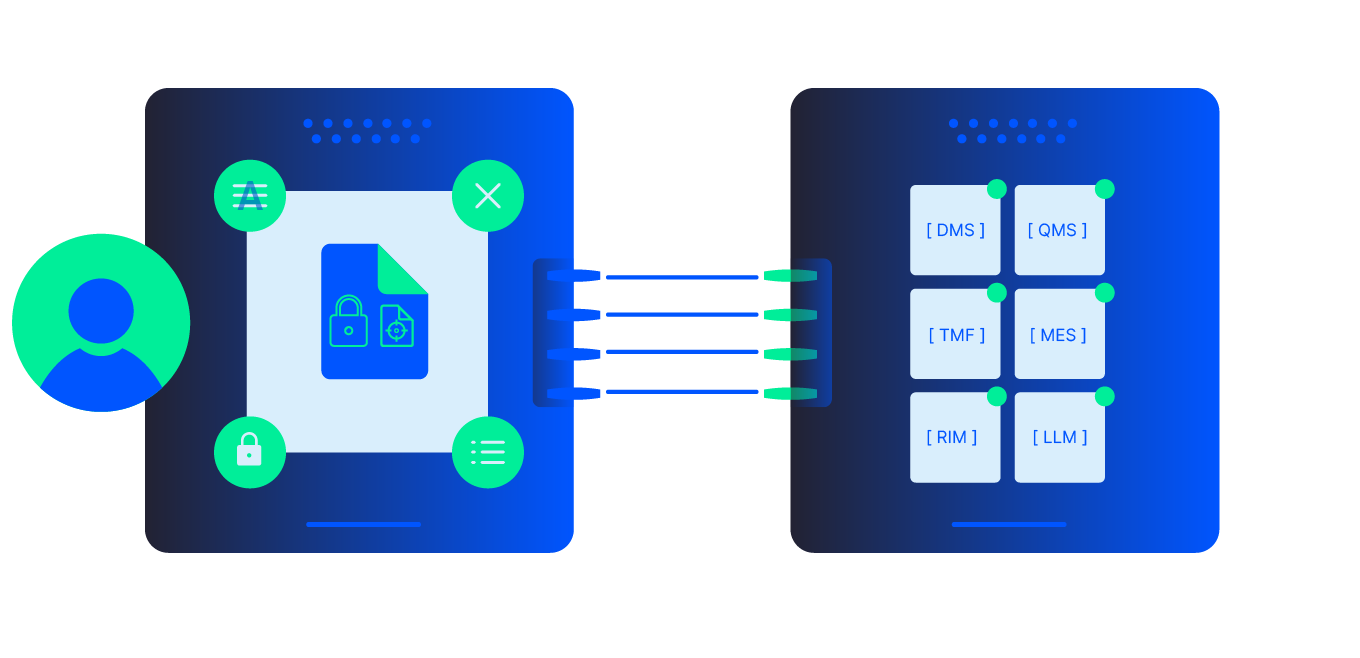
Auto-ingest and standardize trial content into compliance-ready, structured datasets; validate for completeness and priority fields; and assemble audit-ready TMF and eTMF binders, cutting cycle times, reducing audit findings, and improving AI readiness across TMF, CTMS, and DMS.








.jpg)

